The simulation program HySS was developed as part of the Hyperquad suite as a tool to enable good experimental conditions to be identified. The requirements are
The chemical model(s) used in the simulation consists of the chemical species which one supposes might be present and usually includes species whose stability constants are known as well as those that are not known. Estimation is discussed elsewhere. The experimental procedure embraces what chemicals are to be used and what apparatus is to be used.
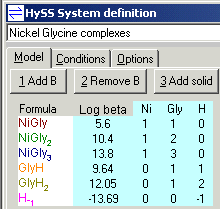
To illustrate the use and value of simulation consider the complexes formed between Ni2+ and the glycinate ion in aqueous solution. Suppose we expect 3 complexes to be formed. The model is then entered into HySS as follows.
Notice that in addition to the 3 unknown stability constants there are ligand protonation constants which are "known", that is, have been previously determined and also a self-dissociation constant for water.
These are a set of conditions chosen for the simulation.
The initial volume depends on the pipette(s) used to put the reagents into the titration vessel. The total millimoles are the amounts of reagents placed in the titration vessel. Notice that the amount of hydrogen ion is the same as the amount of glycinate. This implies that the substance added to the titration vessel is glycine, the protonated form of the glycinate ion. The burette, according to this table, contains alkali (this is the significance of the minus sign) at a concentration of 0.1 mol dm-3. The final volume was chosen so that all the added hydrogen ions would be just neutralized.
The species distribution calculated with these conditions and this model is as follows.
This diagram reveals a very import fact: a single titration will suffice to determine all 3 stability constants. Even though the metal to ligand ratio is 1 to 3 both the 1:1 and 1:2 complexes are formed to an appreciable extent over a good part of the titration range. Naturally the 1:3 complex is also formed. So, in this case the simulation serves not only to establish the experimental conditions but also shows that it is not necessary to perform titrations with metal : ligand ratios of 1:1 and 1:2. (in other cases such titrations may be necessary).
The ideal conditions for a stability constant determination are that the concentration of a species should range between about 5% and 95% of the total amount of that substance. This not always possible. Indeed the diagram above shows that, at the concentrations chosen the maximum extent of formation of NiGly3 is about 80% because of the simultaneous presence of NiGly2 (20%).
The real difficulty is with the so-called minor species. These are species whose formation extent is never large. Simulation can help to see if there are conditions in which the minor species' concentrations can be maximized.
Contents > Planning: Preliminary | HQD file |Estimation | Simulation